Abstract of Special Research Report (RR-24)
National Institute of Occupational Safety and Health, Japan
Study on Maximum Safe Gap with Propane/Hydrogen with Air Mixtures
| RR-24-1 |
| Heizaburo TSURUMI |
: According to the study on the measurement of experimental safe gap with Propane/Hydrogen with air, the behavior of safe gap of this mixtures that is very few reports which give scientific support to at present is concluded clearly as to be discussed in this paper together with each chapter of this report. |
Some Studies on Combustion of Irradiated Polyethylene
| RR-24-2 |
| S. MORISAKI |
: Oxidative thermal degradation products of polyethylenes at various temperatures crosslinked with electron beams have been analyzed with gas chromatography and mass spectrometry techniques. Carbon monoxide and carbon dioxide are determined at a temperature range of 200-340 °C, and the activation energies of the unirradiated and the irradiated polyethylene (at 100 Mrad) are 13.5 and 11.4 Kcal/mole, respectively. C1 to C8 hydrocarbons produced in air and in nitrogen are determined at temperatures from 400 to 540 °C for the polyethylenes. The irradiated polyethylene produces less hydrocarbons in air than the unirradiated polyethylene, contrary to the fact that the crosslinked polymer evolves more hydrocarbons than the unirradiated polymer in a nitrogen atmosphere. Aldehydes and ketones are observed in the volatile oxidative degradation products, and these carbonyl compounds increase quantitatively with increase of temperature up to about 460 °C. It is concluded that irradiated polyethylene is thermally more unstable in the absence of oxygen and more easily oxidable at low degradation temperatures in air than unirradiated polyethylene. Irradiated polyethylene, however, is more heat-stable than unirradiated polyethylene from the standpoint of the ignition process. |
A Quantitative Model of Industrial Accident --An Assessment of Industrial Risk Based on a Method of Nominal Multivariate Analysis using Conception of Entropy and it's Actual Example of Analysis--
| RR-24-3 |
| Yoshinobu SATO, Taiji KONDO, Soichi KUMEKAWA, Noboru SUGIMOTO, Ikuo Mae and Eiki YAMANO |
: In this paper we deal with a method relating to quantitative assessment of a certain risk and show an example making use of it. 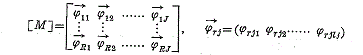
J is the number of the factors. l j is the number of the categories in the j -th factor. φ rjk is defined as follows. 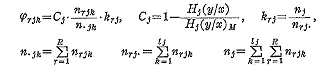
n rjk is the number of the data which replied to the k -th category in the j -th factor and to the r -th event. H j(y/x) is the conditional entropy of the events (which indicate the degrees of damage) under the condition that we are informed of a category in the j -th factor. H j(y/x)M is the maximum value which H j(y/x) can takes when the distribution of the data in each category are changed. The vector e i is expressed as follows. 
e ij is defined as follows. If i -th datum replied to the k -th category in the j -th factor, the i -th component of e ij is equal to 1 and the other components are O. As a result of study in the analysis of actual destructions of grinding wheels, it became possible to evaluate the risk of grinding operation system in some degree. |
Smouldering Temperature of Combustible Dust Layer
| RR-24-4 |
| T. MATSUDA and M. NAITO |
: A layer of combustible dust on a hot surface may ignite if the temperature of the surface is sufficiently high. Although a considerable amount of experimental investigation by many workers have been carried out, the method has not been defined of measuring the minimum temperature, at which smouldering combustion, rather than ignition itself, occurs. The temperature leading to smouldering were taken to be the smouldering temperatures of the dust layers. |
Interruption of Explosions by Flame Arresters (4th Report) -Factors Affecting on Flame Quenching by Wire Gauzes (1)
| RR-24-5 |
| Toshihiro HAYASHI |
: Wire gauges have been considered as one of the useful quenching elements for flame arresters because of their availability, economical cost, low flow resistance and ease of processing, but the experimental evidences are not yet satisfactory. |
Interruption of Explosions by Flame Arresters (5th Report) -Factors Affecting on Flame Quenching by Wire Gauzes (2)
| RR-24-6 |
| Toshihiro HAYASHI |
: As flame arresters are safety barriers to minimize the development of flames after explosions started in closed systems, their failures are deadly, and their performances should have been verified through field tests, if possible, or at least equi-scale explosion tests. |
Safety for Electrical Equipment under Artificial Environments(2nd Report) Ignition of Flammable Solid Materials by Low Voltage Inductive Sparks
| RR-24-7 |
| Ryuji TANAKA and Kenji ICHIKAWA |
: In a hazardous area where an explosive gas be or may be present, the technique of "intrinsic safety" has been applied all over the world. In this paper, the authors aim at applying the technique to designing of safe low voltage circuits which are incapable of igniting a flammable solid in "Oxygen-hazardous Areas". |
Test-Manufucture of Artificial Fingers (2nd Report) --Winslow's Effect in Ionic Exchange Resin Disperse System--
| RR-24-8 |
| Noboru SUGIMOTO and Taiji KONDO |
: Winslow's effect is defined as an essentially instantaneous reversible change in apparent viscosity when a fluid is subjected to an externally applied electric field. |
The Critical Energy for Direct Initiation of Spherical Gaseous Detonations
| RR-24-9 |
| Hidenori MATSUI |
: The paper reports experimental results on the critical energies for direct initiation of spherical detonations using electrical sparks under various electrode geometries and spacings. The results indicate that for large spacings the detailed electrode configurations have no influence on initiation energy. The critical energy per unit length reaches a minimum asymptotic value corresponding to the value found previously for cylindrical detonations. For electrode spacing less than the characteristic explosion length, the electrode geometry has an effect on the initiation energy. The flanged and the pointed needle electrodes from the lower and upper bounds respectively for the critical energy for various electrode configrations. The results indicate that the effects of the electrode geometry are essentially those corresponding to the severity of gasdynamic expansion generated. The gasdynamic effects fall between the cylindrical and spherical symmetries. This paper also describes some new results for the critical energy for direct initiation of detonations in acetylene-oxygen mixtures using flanged electrodes. The present spark energies represent the true effective initiation energies, independent on discharge characteristics and electrode configurations and gap spacings. |